ABOUT IONS...
- Duygu Kurtuluş
- May 28
- 6 min read
Water is the best and safest solvent in the world. We have discussed the solubility properties and chemistry of water in detail in our previous articles related to water. In this article, we will look at the effects of ions on the solubility chemistry of water from a broad perspective. When ions dissolve in water, the electrolytic properties of the solution increase and the electrical conductivity of the water rises. This electrical conductivity gives the water new properties. Ions interact with water molecules to form a hydration layer, which enables more ions to dissolve in the water. Therefore, the increased electrical conductivity enhances water's solvency, allowing it to dissolve more substances.
In their studies on coffee and water, Lockhart and Pangborn attempted to reach the concept of the "ideal" while not ignoring undesirable metals and compounds in coffee such as transition metals, halogens, nitrates, sulfates, and phosphates. However, with where specialty coffee has reached today, we are no longer concerned with halogens, but rather with controlling the amount of even the most beneficial salt ions such as calcium and magnesium. For this reason, as we announced in our previous article, we developed the FINAL TOUCH product. The true trick in coffee brewing is for the solvent to extract the desirable compounds and leave the rest behind in the filter.
Quantifying the role of dissolved ions in the solubility of coffee components is experimentally difficult because there are many competitive interactions—such as ions being dragged from the coordination spheres of water molecules to form ion pairs—that are both entropic and thermodynamically significant. These interactions vary depending on the type of interaction between the dissolved ions and water. For example, hydration of Ca²⁺ is more exothermic than that of Mg²⁺. Dissolution of larger molecules is more complex because solutes often have a complex array of polar motifs. In flavor chemistry, organic compounds exhibit complex behaviors with hydrophilic and hydrophobic regions that interact with water through hydrogen bonding, Coulombic interactions, and the formation of structured hydration cages. In any case, as long as solutes are below saturation, they do not significantly alter hydrogen bonding or electrostatics in the broader system.
In line with Lockhart’s findings, the upper limit of dissolved ions in coffee extraction is not constrained by saturation but by over-extraction. Therefore, by referring to the literature, we will focus on the role of dissolved cations in the extraction of coffee components. We will try to summarize the topic through a specific study. The study proposes an accessible quantum chemical approach to measure how familiar dissolved metal ions—Na⁺, Mg²⁺, and Ca²⁺—bind with coffee organics.
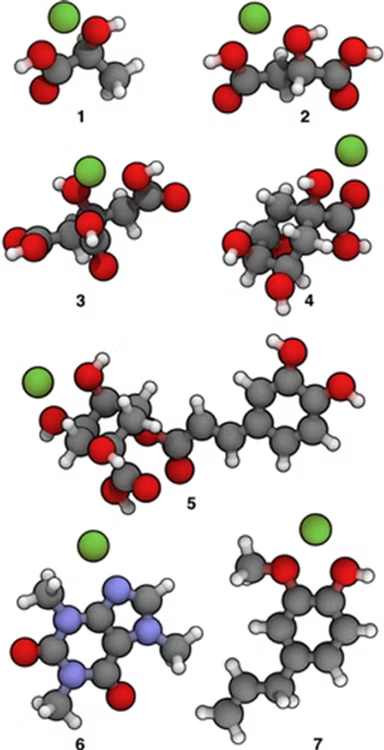
As representatives of organic acids commonly found in roasted beans, the following compounds were selected whose chemical structures are shown in the figures below: five derivative acids (1–5), caffeine (6), and eugenol (7). Among the five acids, lactic acid (1) and malic acid (2) represent sour notes, while citric acid (3) has a sweeter flavor profile. Quinic acid (4) and its larger derivative chlorogenic acid (5) are considered bitter and unpleasant. Caffeine (6) is included as an example of an aromatic alkaloid, while eugenol (7) is known for its pleasant woody aroma found in coffee, wine, and whiskey.

Dissolved cations (Cations are positively charged ions. They become positively charged by losing one or more electrons from atoms or molecules; the salts used in the FINAL TOUCH product exist as cations in aqueous solution) interact with the nucleophilic motifs of dissolved coffee components. A brief note on this positive charge—the cation: the positive charge is very important because most aroma compounds in coffee become negatively charged when dissolved in water, which means they are attracted to positively charged metal ions.
This interaction can be explained by classical electrostatics: here, the interaction energy Ur is proportional to the product of the ion and solute charges (qi and qs) divided by the square of the interatomic distance. Therefore, species with more localized charges are expected to interact more strongly with molecular multipolar components. In short, electrostatic interactions are determined by the charges of the ion and solute and the distance between them.
The 3D visualizations of the organic compounds mentioned above are shown on the left. Ions with more localized charges are expected to show stronger interactions with molecular multipolar components because these components have more widely distributed charges. In the figure on the side, we can see that compounds 1-2-3-4 have a more localized (or “non-diffuse”) morphological structure.
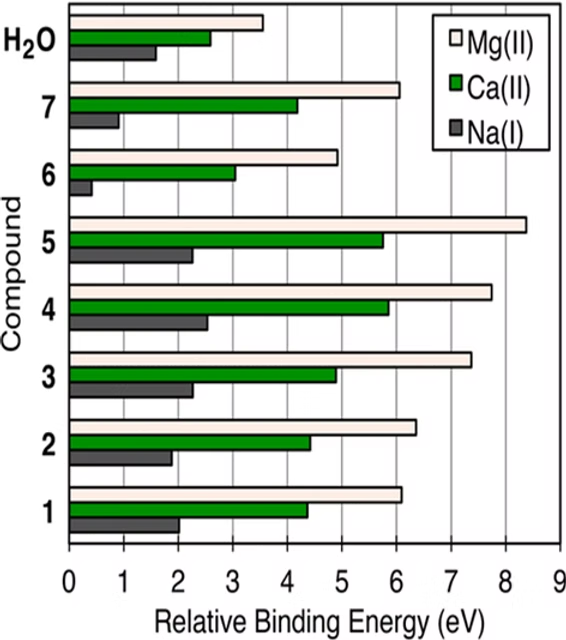
The summary of the binding energies (relative binding energy; the binding energy of one compound relative to another) is shown in the adjacent figure, and the H₂O-M⁺ interaction is included in the table as a reference. The displacement of metal-coordinated water is a process driven by entropy, but the compounds competitively replace the metal-coordinated water molecules. Coordination and interaction require a higher activation energy than that of H₂O-Mn⁺. The table shows which cation is a more effective “partner” for the extraction of the five acids.
Similar binding trends are observed for Ca²⁺ as well; however, in all cases, the relative binding energy is lower compared to Mg²⁺. The binding energy of 6 (caffeine) with Ca²⁺ is comparable to H₂O-Ca²⁺, which is the only example of weak interaction between a divalent cation and an electron-dense motif. Because caffeine’s electron density is spread along its conjugated aromatic motif, low binding energies are obtained for all metal ions (almost no interaction with Na⁺). Based on the table, the results clearly show that binding energies are directly proportional to the charge and inversely proportional to the ionic radius. Therefore, there’s no surprise in K⁺ binding, as its activation energy is expected to be lower than Na⁺ due to its size. To summarize the table more simply: this computational modeling study presents an experiment that calculates the binding energy between seven different coffee flavor compounds and magnesium, calcium, or sodium ions. The results show that all these ions, when binding, increase the extraction of the seven different aroma compounds. Magnesium has the greatest effect in enhancing solubility, calcium slightly less, and sodium-potassium the least. Although each ion has a different effect, panel tests show that the balance of these effects is approximately equal. The Mg²⁺ ion forms the strongest interactions with coffee compounds and ensures the greatest extraction of components from the beans. Additionally, Ca²⁺ also provides a good result, though its interaction strength is lower than Mg²⁺. Na⁺ and K⁺ ions, on the other hand, surprisingly form weak interactions with neutral compounds. As previously mentioned, if the ratio of calcium to magnesium is not well-balanced, they can harden the water, thereby reducing rather than enhancing solubility.
Carbonate is not a cation or metal. It acts as CO₃²⁻, which is a negatively charged group of carbon and oxygen atoms. In coffee-related water discussions, terms like “alkalinity” or “buffering” all refer to carbonate.
The important property of carbonate for us is its ability to absorb acid—meaning hydronium or hydrogen ions. Carbonate can bind acid by converting it into HCO₃⁻ or H₂CO₃ and can release it again when the acid concentration decreases. Therefore, carbonate is called a “buffer” because it buffers against changing acid levels. Since the sodium and potassium sources in our FINAL TOUCH product are provided in bicarbonate forms, they not only supply sodium and potassium ions to the water but also serve the buffering role mentioned above.
The study shows that the ideal solubility rate of coffee beans is determined by both the ionic composition of the water and the amount of bicarbonate.
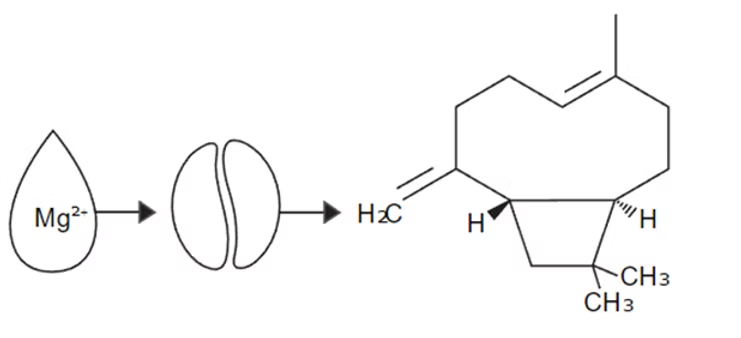
You can see a reaction above in which a coffee compound interacts with a magnesium ion. It’s one of the clearest and most demonstrable examples of how magnesium in water binds to coffee’s flavor compounds and releases them into the brewed coffee. The product of the reaction (the heterocyclic structure on the far right, named caryophyllene) is a compound that contributes a rose-like flavor to your cup.
In the sensory panel of the study we referenced in this post, the tasters were first served a filter brew prepared with "soft, no added-ion" water, which was described as “good” with “slightly sour acidity.” Then, another brew was prepared using water with high calcium content but the same Total Dissolved Solids (TDS) value. Maxwell noted that in this case, the high buffering capacity masked other flavor notes. Finally, a brew made using water ionized with “all the ions mentioned and used in our FINAL TOUCH product” was evaluated. The panel concluded that this brew, where ions were balanced at optimal levels without interfering with each other or causing any salt precipitation, yielded the best cup. The magnesium ions in the water were found to enhance sharp, fruity notes, calcium emphasized heavier, creamy notes, and buffering substances acted antagonistically against sharper, acidic flavors.
The study concluded that the ideal extraction rate of coffee compounds is determined by the ionic composition of the water and the amount of bicarbonate present.
Even though we’ve only shed light on a small part of it scientifically, coffee continues to push us beyond “ideal” or “correct” definitions and instead encourages us to explore just how much more is possible. It seems our journey is far from over.
Duygu Kurtuluş
Co-Founder / Chemist / Nanotechnology Engineer / Hazardous Chemical Consultant / Chemical Evaluation Specialist





Comments